By embedding a silver catalyst inside a porous crystal, KAUST researchers have improved a chemical reaction that converts carbon dioxide (CO2) into carbon monoxide (CO), which is a useful feedstock for the chemical industry.
Carbon monoxide is a building block for producing hydrocarbon fuels, and many researchers are searching for ways to produce it from CO2, a greenhouse gas emitted by burning fossil fuels. One strategy involves using electricity and a catalyst to drive a so-called CO2 reduction reaction. But this reaction typically produces a variety of other products, including methane, methanol and ethylene. Separating these products significantly raises the cost of the process, so researchers hope to guide the reaction to generate a single product.
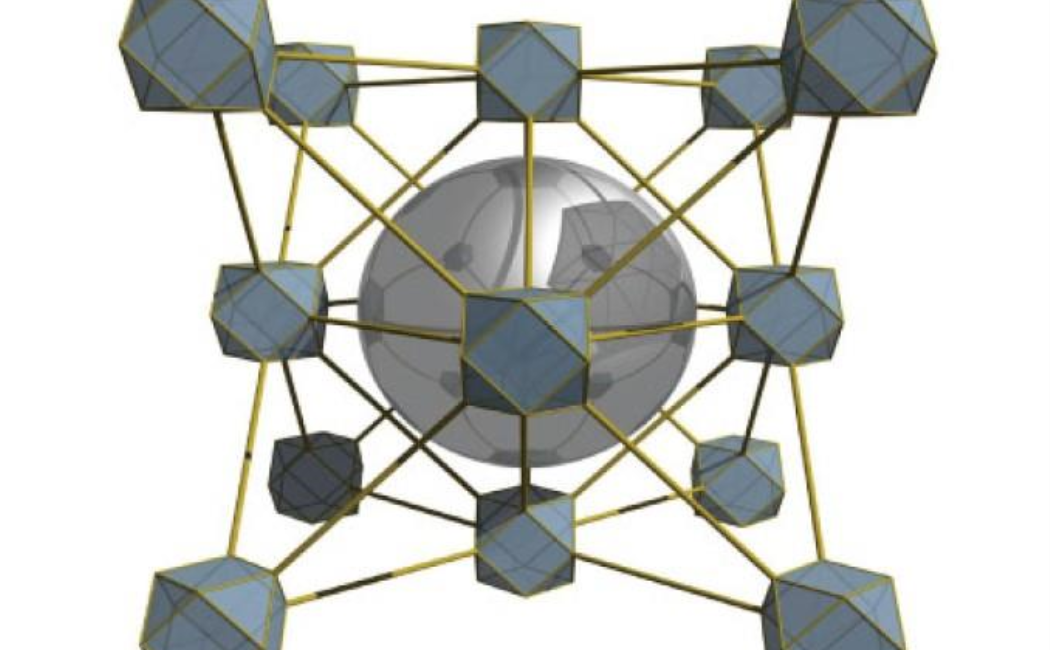
Osama Shekhah and Mohamed Eddaoudi, chemists at KAUST, in collaboration with Ted Sargent’s group at the University of Toronto, have now fine-tuned the CO2 reduction reaction using metal organic frameworks (MOFs). These porous crystals contain a lattice of metal-based nodes connected by carbon-based linker molecules. By altering these components, researchers can tailor the size of an MOF’s pores and its chemical properties.
The researchers created four different MOFs with the same overall lattice arrangement and grew 5-nanometer-wide nanoparticles of silver inside the pores of each MOF. Then they tested each MOF to find how its structure affected the CO2 reduction reaction. They monitored which products emerged from the process and studied how an activated form of CO — a crucial intermediate in the reaction — bound to the silver catalyst.
The most effective MOF contained zirconium-based nodes connected by molecules of 1,4-naphthalenedicarboxylic acid. Because it has smaller pores, its ability to trap CO2 outperformed its rivals.
The silver nanoparticle in this MOF also bound activated CO in a different way than the others, connecting in a “bridging mode” involving two bonds rather than one. This ensured that CO was less likely to transform into unwanted byproducts. “Controlling the type of the CO intermediate during the reaction has a big influence on the CO selectivity,” says Shekhah. Together, these effects boosted the efficiency of CO production to 94 percent, a dramatic improvement in selectivity.
The researchers hope to build on their strategy, making further tweaks to the MOF’s structure to enhance the CO2 reduction reaction. “We believe that this work paves the way for using MOFs as new supports for improving the activity and product selectivity of the CO2 reduction reaction by directly interacting with the gaseous intermediates and controlling their binding mode,” says Eddaoudi.